
Last April, a study by Curative, KorvaLabs, and UCLA showed that Curative’s test detected more cases of COVID-19 infection using the mouth swab method, but there were also three cases found using the nose swab method that hadn’t been found in the mouth swab samples. Credit: Sarah Belle LinĬurative’s testing kit instructs individuals to first cough deeply several times, and then swab around the inside of their mouth. Curative operated a pop-up COVID-19 test kiosk in Berkeley in July. According to California Department of Public Health data, Curative has processed an average of 142,196 tests per week, 14% of its goal. The company’s goal has been to scale up to one million tests every week. that was established by KorvaLabs, a company Curative acquired in May 2020. Curative operates three labs: one in Southern California, a lab in Austin, Texas, and an FDA COVID-19 certified test lab in Washington D.C.

The company has maintained an average turnaround time of 36 hours. In June, Curative’s CEO, Turner, told Bloomberg that his company was conducting roughly 25% of all tests in California. For these reasons we are deferring to the FDA and their public announcement.” “While the FDA’s announcement is concerning, we have no information to suggest these issues with Curative pose a wider threat to public health in Alameda County. “We are aware of the FDA announcement regarding Curative COVID-19 testing,” the Alameda County Department of Public Health wrote in a statement to The Oaklandside today. It’s not immediately clear how many COVID-19 tests have been conducted by Curative in Oakland and Berkeley. Testing sensitivity and accuracy on behalf of our patients is at the heart of our work,” he said. The company’s CEO, Fred Turner, defended Curative’s testing method: “Regarding the FDA safety communication noting the risk of false results with the Curative SARS-CoV-2 test, we are confident in our data and we are working with the FDA closely on the matter. In a patient fact sheet, the CDC warned that “it is possible for this test to give a negative result that is incorrect (false negative) in some people with COVID-19.”Ĭurative responded to the FDA’s announcement with a statement that they’re working with the agency to address its concerns. The Centers for Disease Control and Prevention (CDC) has also acknowledged the false negative issue. This is the first time the FDA made an announcement about false-negative tests since the pandemic began, according to its archive. According to the FDA, false-negative tests can delay medical treatment for an infected person and result in the spread of COVID-19.Ĭurative’s test was never FDA-approved, but the agency did authorize it for emergency use in June 2020 during the pandemic. A false negative means that a test result shows a person doesn’t have COVID-19 when they do in fact carry the virus. On January 4, the Food and Drug Administration, the federal agency in charge of ensuring the safety and efficacy of drugs and medical devices, announced that Curative’s tests may be producing false-negative results. Curative still operates test sites in Oakland, Berkeley, San Francisco, San Mateo, and Palo Alto, and according to Politico, it has tested members of the U.S.
#Curative covid test new braunfels free#
The company-which offers a less physically uncomfortable testing method, relying on a mouth swab instead of a deep nasal cavity swab-opened a free pop-up test site in Berkeley last July, and another test site in Oakland in December.
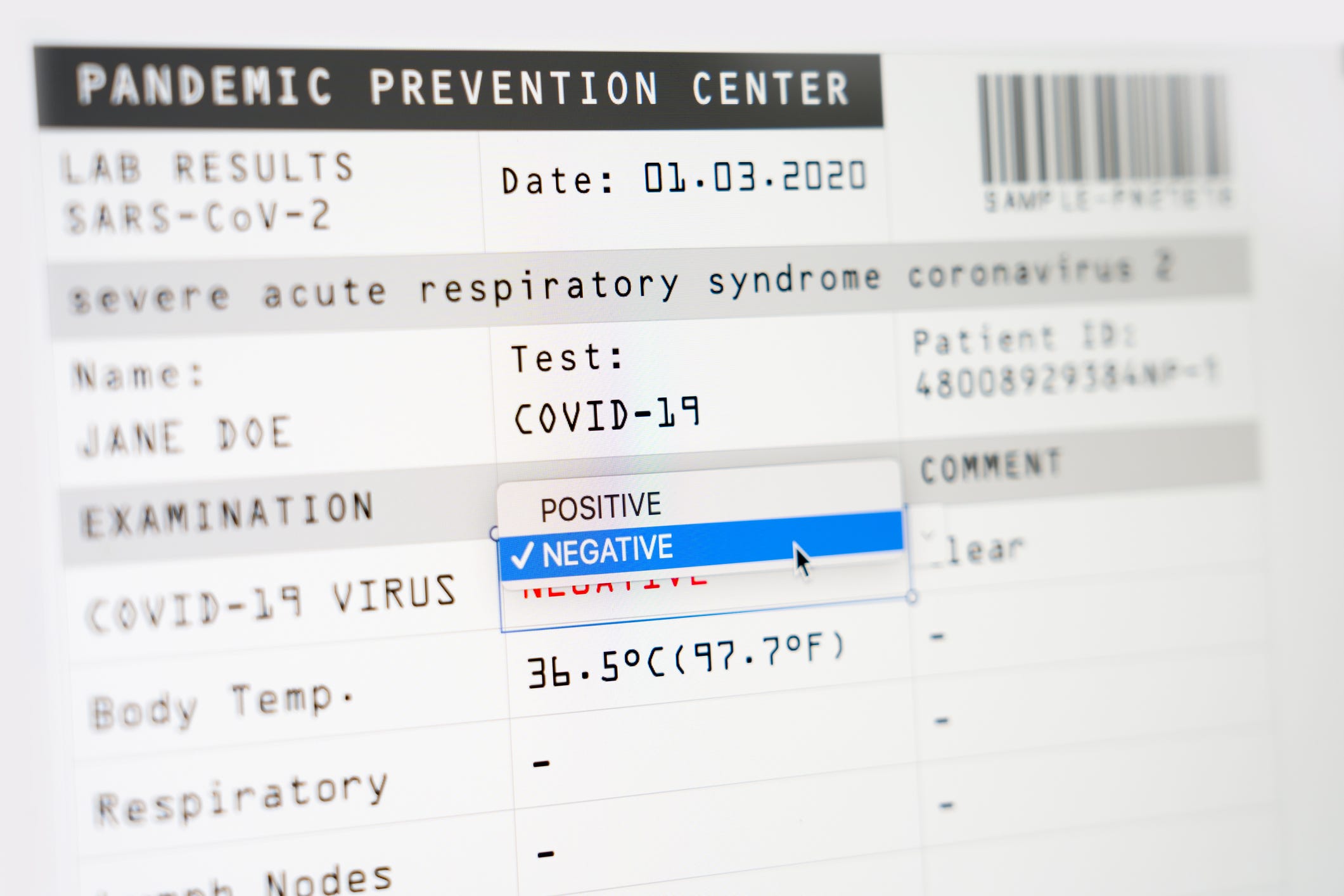
Questions are being raised about the accuracy of a large number of COVID-19 tests processed in Oakland, Berkeley, and much of California.Ĭurative, a healthcare startup founded in January 2020, leapt into action when the pandemic began with promises that it would test millions of Americans.


 0 kommentar(er)
0 kommentar(er)
